Use of PEG to induce and control plant water deficit in experimental hydroponics’ culture.
By A. Blum
Polyethylene glycol (PEG) is a polymer produced in a range of molecular weights. In 1961 a paper published in ‘Science’ (Lagerwerff et al., 1961) indicated that PEG can be used to modify the osmotic potential of nutrient solution culture and thus induce plant water deficit in a relatively controlled manner, appropriate to experimental protocols. It was assumed that PEG of large molecular weight did not penetrate the plant and thus was an ideal osmoticum for use in hydroponics root medium.
During the 1970’s and 1980’s PEG of higher molecular weight (4000 to 8000) was quite commonly used in physiological experiments to induce controlled drought stress in nutrient solution cultures. Several papers also reported theoretical or measured concentration-osmotic potential relations for PEG of different molecular weights (e.g. Money, 1989; Michel, 1983; Michel and Kaufmann, 1973). Experience gained by users indicated that these relationships can diverge to some extent depending on the lot or source of the specific PEG used. It is therefore advisable to measure the actual osmotic potential of the solution culture containing PEG.
Calculation of the osmotic potential of aqueous PEG solutions is given here only for PEG 6000 and 8000 which are used in plant experiments. Standard nutrient solutions containing PEG are practically very similar to pure aqueous solutions. The calculations are presented in the following MS-Excel sheet (download here to your computer)
Problems in using PEG
PEG uptake by plants
Even PEG of high molecular weight, such as 4000 to 8000 was found to be taken up by plants. PEG was found in shoots and roots. PEG was taken up by maize and bean plants at a relatively slowly rate of 1 mg/g fresh weight per week. However, when roots were damaged or broken, rate was higher. Cotton absorbed less PEG (Lawlor, 1970). Pepper plants also took up PEG, where the higher molecular weight PEG was mostly concentrated in roots while the lower molecular weight compounds accumulated in leaves (Janes, 1974). Yaniv and Werker (1983) presented striking photographs of PEG 1500 to 6000 mw deposited on leaves of various solanaceous plants exposed to PEG in the root medium for 24 h or less. Again, greater deposition was seen in plants with physically damaged roots. PEG 6000 was taken up by tomato plants and found in older leaves and roots (Jacomini et al, 1988). The critical finding was that leaves containing PEG behaved hydraulically differently from leaves without PEG when grown in PEG containing nutrient culture. It can therefore be concluded that PEG, even of higher molecular weight, is taken up by plants and the rate of uptake and concentration in shoots and roots depends on the species, on PEG concentration, on time of exposure and on root damage.
Hypoxia in PEG solutions
Verslues et al. (1998) reported that plants grown in nutrient culture containing PEG suffered from hypoxia and if such a system is used the solution should be oxygenated.
Mineral contamination of PEG
PEG of 3000 to 4000 mw from two commercial sources was found to be contaminated by high concentrations of phosphorus, which could introduce a problem if used in experiments involving 32P or P interactions (Reid, 1978). Toxic metals such as aluminum were also found to contaminate PEG and pose a toxicity problem when PEG was used in culture (cited in Reid, 1978).
Can PEG be used as a legitimate osmoticum in nutrient solution culture?
PEG uptake
Before PEG culture is attempted, the specific plant species should be tested for the extent of PEG uptake into roots and leaves. Different methods of analysis are available. The simplest and probably the one sufficient for this purpose is the turbidimetric method based on precipitation by trichloroacetic acid (Lawlor, 1970; Janes, 1974). High pressure liquid chromatography (Yaniv and Werker, 1983) has also been used. Reduction in uptake can be achieved by avoiding any root breakage during plant handling and culture.
Finally, plants can be grown in PEG containing nutrient solution using semi-permeable membranes to separate the roots from the PEG solution (Tingey and Stockwell, 1977). With this system the plants are grown in a “container” made of semi-permeable membrane (such as “Spectra/Por” “Standard RC membranes”) placed in a plastic test tube. These tube-like membranes come with different molecular weight cutoff. For example, when using PEG 6000 an available molecular cutoff (MCO) of 3500 will not allow PEG to cross the membrane. Membranes come in rolls of 5-15 m length, depending on their width and specs. A length of the tube-like membrane (equal to double the length of the test-tube) is cut and folded into a sac (Fig.1) and fitted into a plastic test tube which has a hole made in its base (Fig.1). The membrane-tube diameter should fit the test tube diameter. 34mm diameter is the maximum available for MCO3500. Vermiculite is poured into the tubes and tubes are immersed in water in a tray (Fig.1). Seed are germinated in the wet vermiculite. After emergence water is drawn out of the tray and the growing seedlings are allowed to dry the vermiculite to a moderate extent for several days. Then, PEG in nutrient solution is poured into the tray. Water will penetrate the vermiculite but it will be under the negative potential (“suction”) of the PEG outside. It may take up to a week or more to see initial symptoms of plant stress, depending on light intensity and VPD. This slow development of stress is most desirable as it simulates natural conditions. This system requires no aeration because roots are aerated atmospherically by diffusion through the vermiculite. The PEG nutrient solution should be exchanged weekly.
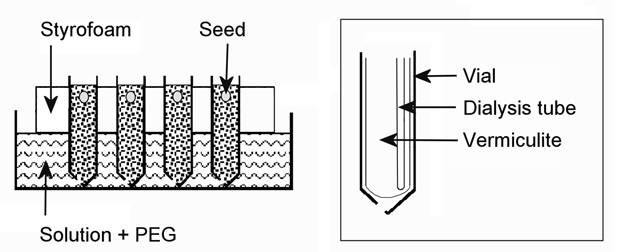 |
A schematic representation of growing plants in PEG6000 nutrient culture without exposing roots to direct contact with PEG as facilitated by use of a semi-permeable MCO3500 spectrapor RC membrane used in dialysis (http://www.spectrapor.com ) (Author’s experiment) |
PEG toxicity
Contaminants contained in PEG can be removed by ion exchange, gel filtration, dialysis or even by recycling PEG through plants (Plaut and Federman, 1985).
PEG hypoxia
PEG solution can be sufficiently oxygenated by aeration, using simple aquarium pumps.
Comment on Mannitol
Mannitol solution or mannitol containing nutrient solution is often used in laboratories as a medium for inducing drought stress in plant and tissue cultures. Mannitol is a natural product that accumulates in certain lower and higher plant species. It should not be surprising that mannitol is taken up by plants when grown in mannitol containing medium (e.g. Fritz and Ehwald, 2010; Lipavska and Vreugdenhil, 1996). Such a system is not suitable for the study of plant response to root medium water status and it will produce artifacts. Plants will respond to stress according to the amount and the effect of mannitol taken up into plant tissues, and not only in response to medium water status.
In conclusion
If you intend to use PEG in nutrient culture, consider the following points:
- Use high mw PEG (6000-8000). Avoid using mannitol.
- Find out or investigate yourself if the plant species you use takes up PEG.
- If needed use semi-permeable membrane system.
- Avoid any root damage.
- Detoxify the PEG you use or at least obtain a mineral analysis of the given lot. Do not use different lots of PEG in one experiment.
- Add PEG to the growing plants in the nutrient solution in increments to avoid plant shock (half and half at 3-4 days interval). This is not needed if the experiment is performed with semi-permeable membranes.
- Measure the final solution osmotic potential. Do not rely only on calculations.
- Cover and wrap the containers so as to minimize exposure of solution and roots to light.
- Aerate the solution and change it once every 4-6 days or as frequently as possible.
- Do not forget to add water to replenish the solution, daily.
- Always measure mid-day leaf water status in the course of the experiment.
Literature Cited
Fritz, M. and Ehwald, R. 2010. Mannitol permeation and radial flow of water in maize roots. New Phytol.188:210–217.
Jacomini E. Bertani A. and Mapelli S. 1988. Accumulation of polyethylene glycol 6000 and its effects on water content and carbohydrate level in water-stressed tomato plant. Can.J.Bot.66:970-973.
Janes B.E. 1974. The effect of molecular size concentration in nutrient solution and exposure time on the amount and distribution of polyethylene glycol Plant Physiol.54:226-229.
Lagerwerff J.V., Ogata G. and Eagle H.E. 1961. Control of osmotic pressure of culture solutions with polyethylene glycol. Science 133:1486.
Lawlor D.W. 1970. Absorption of polyethylene glycols by plants and their effects on plant growth. New Phytol.69:501-514.
Lipavska H. and Vreugdenhil D. 1996. Uptake of mannitol from the media by in vitro grown plants. Plant Cell Tissue.Org.Culture 45:103-107
Money N.P. 1989. Osmotic pressure of aqueous polyethylene glycols. Relationship between molecular weight and vapor pressure deficit. Plant Physiol.91:766-769.
Michel B.E. and Kaufmann M.R. 1973. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 51:914-917.
Michel B.E. 1983. Evaluation of the Water Potentials of Solutions of Polyethylene Glycol 8000. Plant Physiol. 72:66–70.
Reid C.P. Bowen G.D. and McCleod S. 1978. Phosphorus contamination in polyethylene glycol. Plant Physiol.61:708-709.
Tingey D.T. and Stockwell C. 1977. Semipermeable membrane system for subjecting plants to water-stress. Plant Physiol.60:58-62.
Verslues P.E., Ober E.S. and Sharp R.E. 1998. Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol.116:1403-1412.
Yaniv Z. and Werker E. 1983. Absorption and secretion of polyethylene glycol by Solanaceous plants. J.Exp.Bot.34:1577-1584.








